Proteases play essential roles in protein activation, cell regulation and cell signaling, as well as, in the generation of amino acids for protein synthesis or utilization in other metabolic pathways. The inhibition of viral proteases necessary for proteolytic processing of polyproteins has been a successful strategy in the treatment of human immunodeficiency virus (HIV) and hepatitis C respectively, proving the potential of protease inhibitors for the treatment of viral infections. Similarly, the main protease of SARS-CoV-2 (Mpro or 3CLpro) is thought to be essential for viral gene expression and replication and, therefore, is regarded as a major target for anti-CoV drug design. AAT Bioquest offers a variety of dark-FRET peptide substrates and fluorogenic substrates which can be effectively used to screen for inhibitors of coronavirus proteases, papain-like proteases and more.
Covidyte™ COVID-19 Substrates
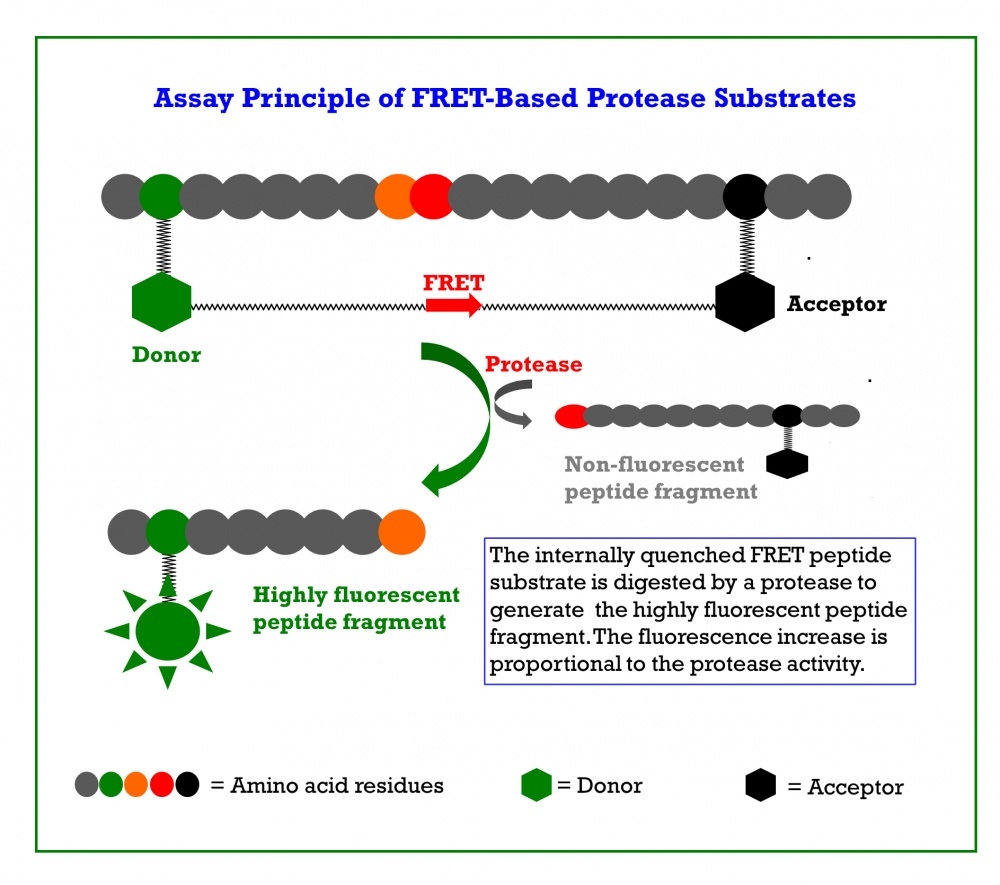
Covidyte™ dark-FRET peptide substrates are robust high throughput screening tools for determining inhibitors of coronavirus proteases. Covidyte™ substrates contain either an 11 or 14 amino acid sequence that can be cleaved by coronavirus proteases. Modified to the terminal ends of each amino acid sequence is a fluorescent donor and non-fluorescent quencher dye. In its intact conformation, substrate fluorescence is quenched by the two dyes being in close proximity of one another. When the internally quenched Covidyte™ substrate is hydrolyzed by coronavirus proteases, a highly fluorescent peptide fragment is produced, and the fluorescence increase is proportional to the coronavirus proteases activity.
Covipyte™ COVID-19 Substrates
The Covipyte™ EN450 dark-FRET peptide substrate contains a 9 amino acid sequence (RELNGGAPI) specific for coronavirus papain-like proteases (PLpro). Conjugated to the N- and C-terminals, respectively, are an Edans donor molecule and a Dabcyl quencher molecule. In its intact conformation, substrate fluorescence is quenched by the donor and quencher molecule being in close proximity of one another. When hydrolyzed by coronavirus PLpro, a highly fluorescent Edans fragment is produced and the fluorescence increase is proportional to the coronavirus proteases activity making it a convenient tool for screening and studying the kinetics of PLpro inhibitors. PLpro of coronaviruses carries out proteolytic maturation of non-structural proteins that play a role in replication of the virus and performs deubiquitination of host cell factors to scuttle antiviral responses.
Dark-FRET Peptide Substrates for Studying Coronavirus Protease Activity
| Product | Length | AA Sequence | Target | Ex (nm) | Em (nm) | ε1 | Size |
| Covidyte™ EN450 | 14 AA | KTSAVLQSGFRKME | Coronavirus proteases | 336 | 445 | 5900 | 100 tests |
| Covidyte™ EN450 | 14 AA | KTSAVLQSGFRKME | Coronavirus proteases | 336 | 445 | 5900 | 1000 tests |
| Covidyte™ ED450 | 12 AA | VNSTLQSGLRKM | Coronavirus proteases | 336 | 445 | 5900 | 100 tests |
| Covidyte™ ED450 | 12 AA | VNSTLQSGLRKM | Coronavirus proteases | 336 | 445 | 5900 | 1000 tests |
| Covidyte™ TF670 | 14 AA | KTSAVLQSGFRKME | Coronavirus proteases | 649 | 664 | 250,000 | 100 tests |
| Covidyte™ TF670 | 14 AA | KTSAVLQSGFRKME | Coronavirus proteases | 649 | 664 | 250,000 | 1000 tests |
| Covidyte™ IF670 | 12 AA | VNSTLQSGLRKM | Coronavirus proteases | 656 | 670 | 250,000 | 100 tests |
| Covidyte™ IF670 | 12 AA | VNSTLQSGLRKM | Coronavirus proteases | 656 | 670 | 250,000 | 1000 tests |
| Covipyte™ EN450 | 9 AA | RELNGGAPI | Papain-like proteases | 336 | 445 | 5900 | 100 tests |
| Covipyte™ EN450 | 9 AA | RELNGGAPI | Papain-like proteases | 336 | 445 | 5900 | 1000 tests |